Supplemental Data to Mesenchymal Stem Cells Transmigrate Between and Directly Through Tumor Necrosis Factor-α-Activated Endothelial Cells Via Both Leukocyte-Like and Novel Mechanisms Stem Cells, 2012 ref. All Detailed Descriptions
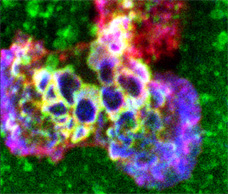
Video 1: MSC induce transmigratory cup formation on endothelial surface.
Detailed Description
Video depicts an MSC interacting with TNF-α activated hLMVEC and corresponds to Fig.2B. Samples were stained for CD90 (green, top left panel), VCAM-1 (red, top right panel), and actin (blue, bottom left; merge of all three channels shown in lower right panel), imaged by serial section confocal microscopy and rendered as a series of 3D projections rotated progressively about the y axis for a total of 360o. Note the formation of a cup-like structure formed by VCAM-1 enriched finger-like projections, which extend from the apical surface of the endothelium up the side of, and seemingly embracing, the MSC.
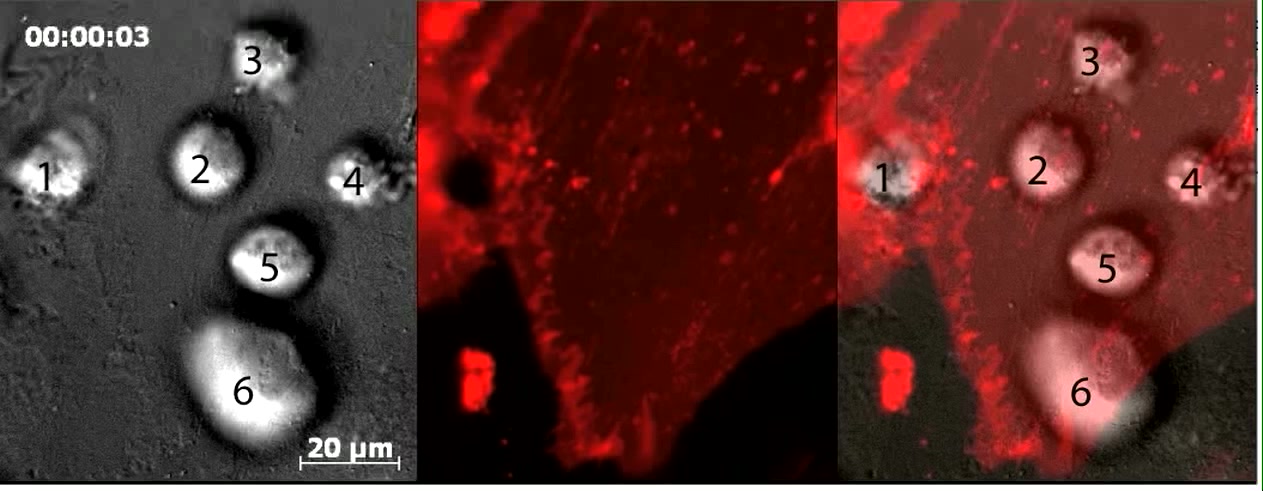
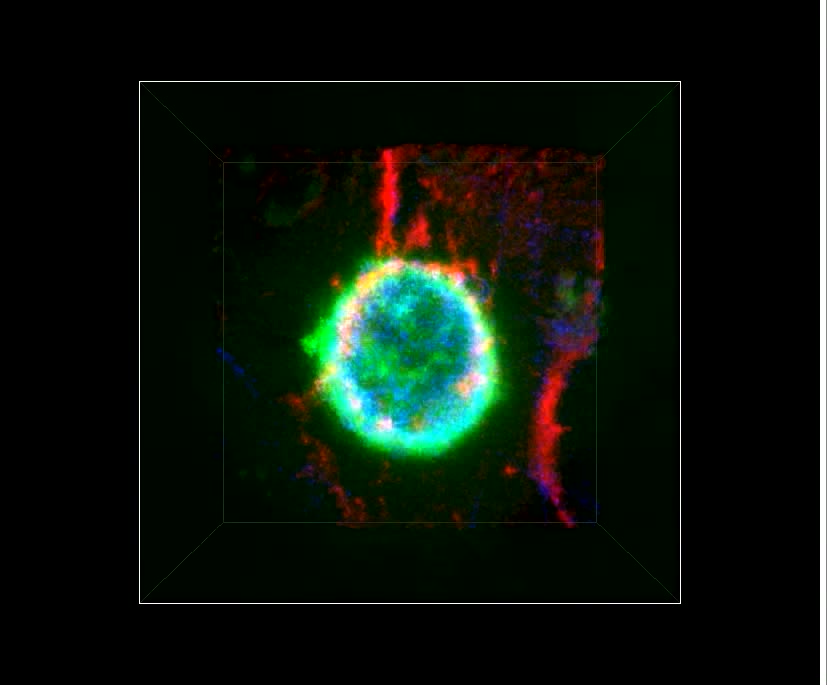
Video 2: MSC transmigrate across the endothelium in the absence of lateral migration.
Detailed Description
Video depicts dynamic live-cell DIC (right and left panels) and fluorescence (middle and left panels, red) imaging of six MSC on memDsRed-transfected, TNF-α activated hLMVEC and corresponds to Fig.3B. Numbers in first video frame identify 6 separate MSC. MSC #1 and #2 are in the process of paracellular and transcellular diapedesis, respectively. Note that for MSC #2 the transmigration pore gradually expands from ~1 μm to nearly 20 μm (see red channel) as it progressively spreads its membrane in the subendothelial space in a starburst-like pattern as seen in the DIC channel (see outline in paused frame at 20:31 time point). MSC #3-6 are apically adherent and have not yet initiated transmigration. Notably, these cells do not display any significant spreading, polarization or net lateral migration over a 45 minute duration. However, these do exhibit sporadic ‘jerky’ motions that seem to correlate with bursts of bleb-like protrusions against the endothelial surface (e.g. orange arrow in paused frame 15:31; see also Videos 3-5). Scale bar represents 20 μm.
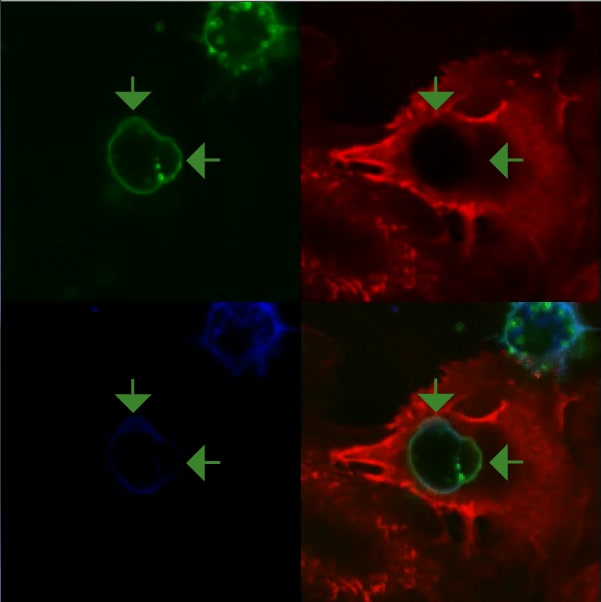
Video 3: MSC exhibit multiple actin-negative and actin positive blebs on their surface.
Detailed Description
Movie depicts serial confocal section (played in series) of a MSC initiating transcellular diapedesis across TNF-α activated hLMVEC and corresponds to Fig. 4A. Samples were stained for CD90 (green, top left panel), VCAM-1 (red, top right panel), and actin (blue, bottom left; merge of all three channels shown in lower right panel). Note multiple highly rounded, bleb-like structures (green; one to several mm in diameter) can be seen protruding from the MCS surface, which are both negative and positive for cortical F-actin (blue, see merged panel, bottom, right). Also note that, in addition to blebs on relatively more apical surfaces, blebs can clearly be seen at the MSC-EC interface, including at and just beneath the transcellular pore (green arrows).
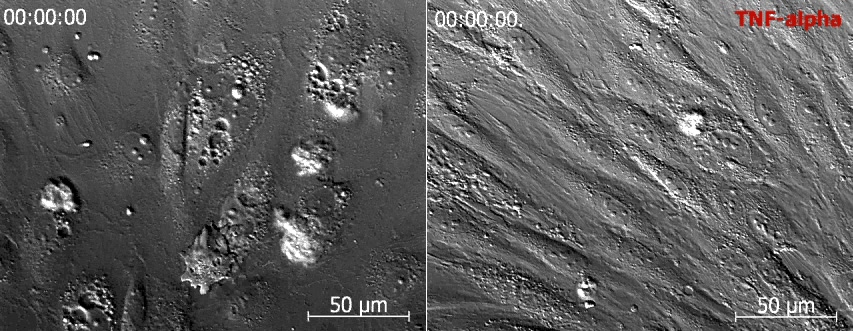
Video 4: MSC exhibit similar blebbing and absence of lateral migration on resting and activated endothelium.
Detailed Description
Left and right panel shows time-lapse DIC imaging of MSC added to resting and TNF-α activated hCMVEC, respectively. No significant difference in blebbing or lateral migration of the MSC is evident. Scale bar represents 50 µm.

Video 5: MSC blebbing may facilitate transendothelial migration
Detailed Description
Forceful membrane blebbing is associated with early stages of MSC adherence, transendothelial gap/pore formation, and subendothelial spreading as shown in a mosaic movie composed of 5 example videos representing progressive phases in the transmigration process.
Example 1: An MSC apically adherent to hCMVEC imaged by high spatial and temporal (10 frames/minute) DIC imaging. Repetitive cycles of large (1 to nearly 10 mm) blebs formation and retraction over all surfaced of the MSC are seen. Individual blebs protruded rapidly, reaching their maximum diameter within an average of 18 seconds and then somewhat more slowly (average duration of retraction phase of 51s).
Example 2: An MSC (DIC, left panel) apically adherent to a memRFP-transfected hCMVEC (red; third panel from left). Note that a large pre-existing paracellular gap is present near, but independent of, the MSC). Interference-contrast reflection microscopy (IRM) is shown in the second panel from the left. IRM reports regions of extremely close contact between cells (i.e., the endothelium) with the underling substrate (i.e., the coverglass) as darkened regions. It was observed that as the MSC formed bleb protrusions against the apical surface of the endothelium dynamic dark spots (with bleb-like spatial and temporal scale) in IRM (e.g., see yellow dashed line in paused frame 1:57). See additional example in Fig.5B. This provides evidence that MSC blebs can exert a force on the endothelium sufficient to locally drive it into closer contact with the underlying basal substrate.
Example 3: An MSC (DIC, left panel) apically adherent near the intercellular junction (see faint vertical line of enriched red fluorescence) of two adjacent memRFP-transfected hCMVECs (red). Note that blebbing activity is associated with initial formation of small paracellular gaps.
Example 4: An MSC (DIC, left panel) apically adherent near an activated GPNT EC intercellular junction formed between a positive memRFP transfected (red signaling in middle panel) and neighboring non-transfected GPNT ECs (black areas in middle panel) in a confluent monolayer. Note that in this example blebs seem to drive a dramatic expansion of a paracellular gap that becomes further distorted and expanded as the MSC begins to migrate further into the subendothelial space.
Example 5: An MSC (DIC, left panel) in late stages of transcellular diapedesis across memYFP-transfected hLMVEC. Over the course of the video MSC progresses from a state of being ~50% below the endothelium, to being nearly completely spread in the subendothelial space (though the pore has not yet closed over the MSC). Critically, this transition is associated with extensive and dynamic membrane blebbing activity both in the apical and subendothelial portions of the MCS. It is noteworthy, that these blebs clearly exert force against the endothelium as evidenced by the induced distortion of the endothelial membrane (green). Indeed, subendothelial blebs protrusion is seen to give rise to transient bright green rings as the push against the basal surface of the endothelium. During the final ~10 min of the video, blebbing gradually ceases as the MSC transition to spreading radially in a more lamellipodia-like fashion. Scale bar represents 10 μm.
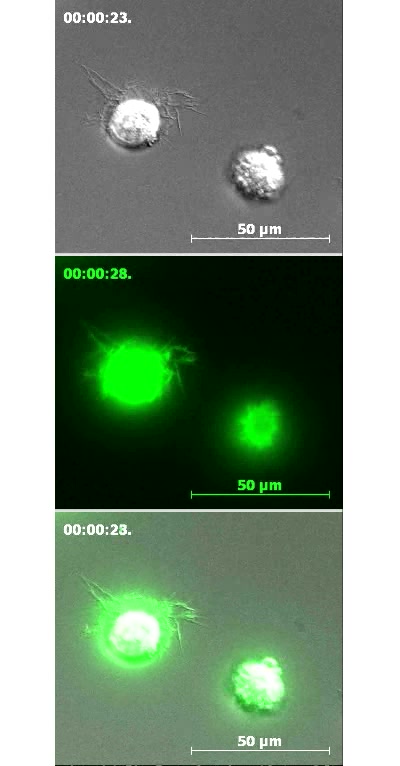
Video 6: Blebbing is specific to MSC interactions with endothelium.
Detailed Description
Movie consists of two distinct segments. In the first segment GFP-actin-transfected MSC were settled on fibronectin-coated glass. DIC is shown in top and bottom panels. GFP (green) is shown in middle and bottom panels. Note that on this substrate MSC display a combination of filopodia and lamellipodia membrane extensions. Although the MSC on the right initially demonstrates some blebbing activity, it quickly switches to the lamellipodial-mediated spreading. In the second segment aortic adventitial fibroblasts have been added to the surface of activated hCMVEC. Note that these cells do not display blebbing activity on endothelium but rather undergo gradual spreading that is coupled to filopodia- or microspike-like membrane protrusions. Scale bars represent 50 μm.
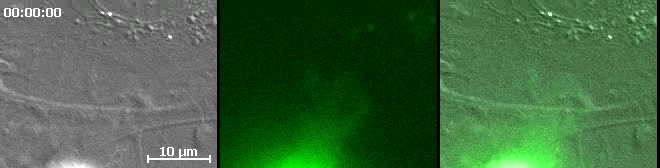
Video 7: MSC exhibit canonical non-apoptotic migratory blebbing dynamics.
Detailed Description
Movie depicts dynamic live-cell DIC (left and right panels) and fluorescence (green, middle and right panels) imaging of an actin-GFP-transfected (green) MSC transmigrating through TNF-α activated hLMVEC and corresponds to Fig.5G. The shown example is of a relatively late stage diapedesis event in which the MCS is advancing part of its membrane under the endothelium. Note that MSC blebs can be seen (via the DIC imaging) protruding from the MSC that are initially are negative for GFP-actin (green), into which GFP-actin is subsequently recruited followed by the bleb finally retraction in a fashion identical to cycles exhibited by some tumor and embryonic cell undergoing non-apoptotic migratory blebbing 16-18. These cycles of membrane protrusion and retraction seem to be coupled to the overall advancement of the MSC laterally (migrating from bottom to top in the video frame) under the endothelium. Scale bar represents 10 μm.
“Video for Everybody” v0.3 by Kroc Camen camen design